
In January 2023, the ICES Kidney, Dialysis & Transplantation (KDT) Research Program celebrated 10 years of championing transformative research across Ontario and Canada. This past decade, we have had the privilege of supporting our researchers and partners using linked administrative healthcare databases. Since its establishment, KDT supported the initiation of 553 projects, published 256 manuscripts, completed 52 reports, and supported over $25 million in awarded grant funding. This decade of research and support has prompted tangible improvements in healthcare, health policy, and research methodology across the spectrum of kidney disease and solid organ transplantation.
KDT’s staff, scientists, leadership, and partners have together worked hard to ensure our research has been impactful. We remain grateful for the trust many put in KDT, to help find answers to important questions.

KDT's Year in Review
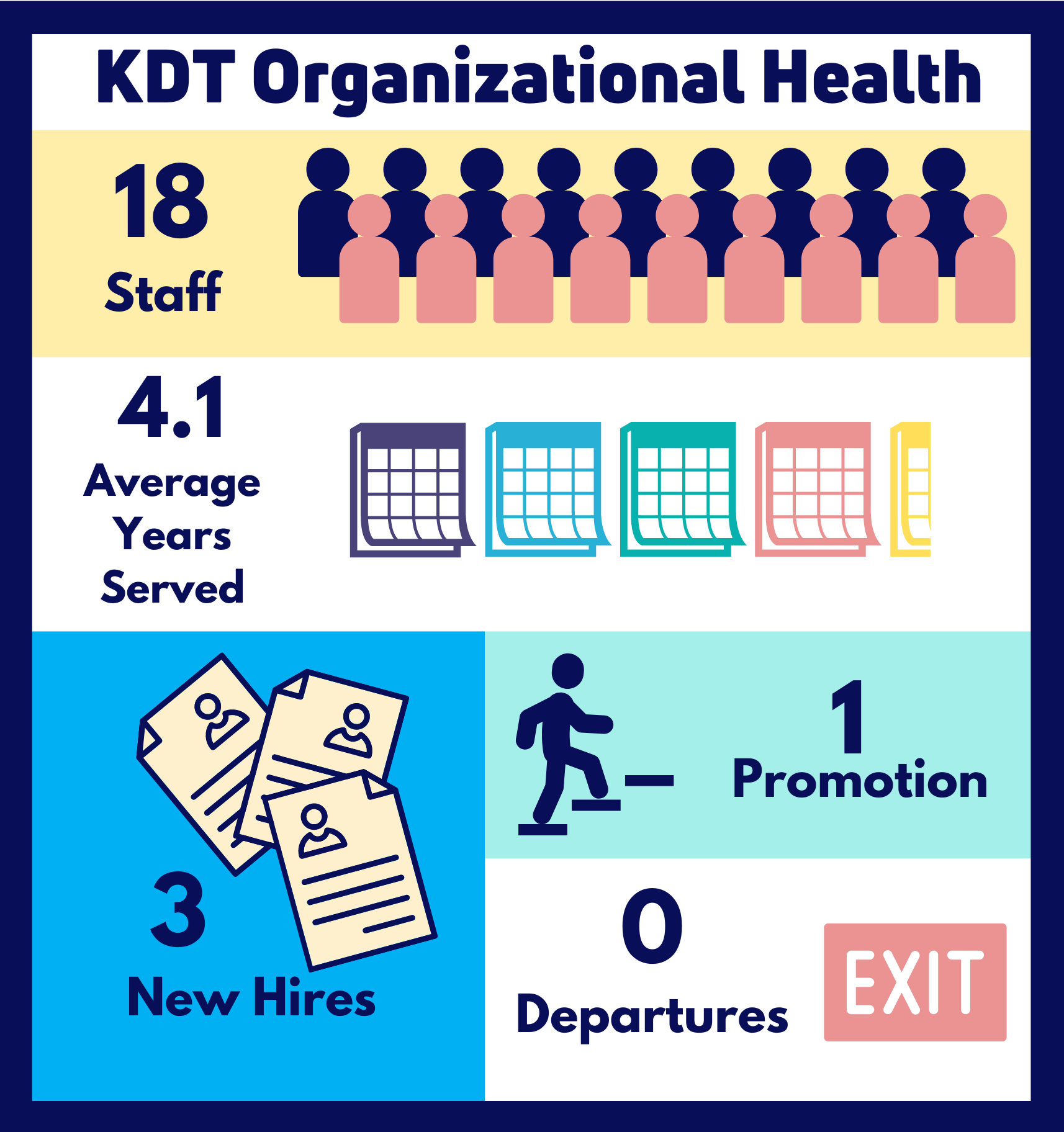
Our 18 staff, 20 scientists, and 18 trainees have applied their expertise to expand the collection of valuable research focused on preventing and treating kidney disease and improving access and outcomes of solid organ transplantation. Through these combined efforts this last fiscal year we initiated 39 projects, completed the analysis for 22 projects, published 47 manuscripts, delivered 4 reports, and supported more than $500,000 in grant funding awarded to our community of investigators.
KDT would like to thank our staff, Sheikh Abdullah, Samar Almadhoun, Sarah Bota, Kathleen Brown-Blake, Steph Dixon, Christina Frederiksen, Nivi Jeyakumar, Greg Kang, Shane Kilburn, Lauren Killin, Bin Luo, Eric McArthur, Taylor McLinden, Danielle Nash, Kyla Naylor, Lima Rodrigues, Graham Smith, and Kathryn Stirling, for their hard work and dedication. KDT is the great success that it is today because of the work they invest into the program and their shared passion for improving the lives of persons living with kidney disease.
*Includes April 1, 2022, to March 31, 2023.
Profiles in Impact

Dr. Amber Molnar's work to improve the lives of patients living with CKD
ICES scientist Dr. Amber Molnar’s uses her research as a robust tool to improve the lives of her patients. Dr. Molnar is a nephrologist at St Joseph's Healthcare, Hamilton, an associate professor with the Department of Medicine, Division of Nephrology at McMaster University, and holds a cross-appointment with the Department of Health Research Methods, Evidence, and Impact.
She is as an adjunct ICES scientist with the KDT Research Program, where she uses large, provincial healthcare databases to improve the quality of care and outcomes in patients with advanced chronic kidney disease (CKD) and in patients receiving maintenance hemodialysis. She has a particular interest in improving the evidence base for how nephrologists prescribe hemodialysis and how patients with end-stage kidney disease navigate their treatment options.
“Most of the decision-making process around when to start and how to treat patients on dialysis is based on what we think is best for that patient, centred around their physiology and what has previously been done,” Dr. Molnar said. “We need to rigorously test the different components of the dialysis prescription to determine what is best for patients to optimize their outcomes.
Managing kidney disease and preparing for dialysis or a kidney transplant requires many decisions to be made with the patients’ care teams that can be hard for patients to navigate. Dr. Molnar’s goal is to reduce the number of patients who start dialysis urgently, which is stressful for patients and is associated with high morbidity and mortality.
“Early on in my training, I was frustrated by the high number of patients, who, despite being well known to a kidney doctor and regularly followed, were admitted to hospital to urgently start dialysis,” Dr. Molnar said. “There is much we can do to help patients better navigate the path to end-stage kidney disease.” To this end, many of her studies focus on improving pre-dialysis care, the transition to dialysis, and shared decision-making around end-stage kidney therapy preparation. This includes projects done in collaboration with the Ontario Renal Network to ensure that pre-dialysis and dialysis care are optimally delivered across the province.
One area that Dr. Molnar identified that had little evidence-based information was opioid prescribing practices for patients with CKD. Chronic pain is a common symptom for patients living with CKD and it is complex to manage. Dr. Molnar and her team examined opioid prescribing across Ontario in a population-based retrospective cohort study using ICES databases, focusing on adults with CKD who received opioid prescriptions from November 2012 to December 2018. They found that opioid use was common in patients with CKD, totaling just under 30% of the 680,000 patients in the cohort, with repeated or long-term opioid use being common. The study concluded that, while opioid prescriptions have declined in recent years, interventions and education on safer practices are needed to improve pain management without using opioids.
Dr. Molnar’s ongoing research to improve the lives of patients living with CKD has and will continue to provide answers and support for improvements in patient care. Our community thanks Dr. Molnar for her dedication both to her patients and to her research, which is more broadly improving the care of all patients living with kidney disease. Her work with KDT has and continues to have a great impact.

Lauren Killin's dedication to advancing KDT research
Lauren Killin, MSc, is an epidemiologist whose primary area of research is the advancement of innovative pragmatic large-scale randomized controlled trials focused on improving healthcare for persons living with kidney disease. Ms. Killin joined our team in August of 2020 after earning her Master’s in epidemiology and biostatistics from Western University and has since become a vital member of KDT.
Ms. Killin manages several projects, assisting and leading the development of data creation and analytic plans, supporting data interpretation and the writing and presentation of trial protocols, peer-reviewed publications, reports, abstracts, and building and translating KDT research. During her time with KDT, she has contributed to approximately 10 KDT projects and five journal article publications.
In late 2020, Ms. Killin added her expertise to the MyTEMP Trial, a pragmatic trial that assessed whether personalizing the temperature of hemodialysate used during dialysis sessions affects cardiovascular-related death and hospitalizations in persons receiving dialysis. Her involvement in the project was pivotal to its success, as she developed the dataset creation plan for the main trial analysis. This plan was used to inform the published statistical analysis plan, which Ms. Killin helped write.
Ms. Killin’s contribution to the analysis enhanced her expertise in epidemiological and analytic methods used in large-scale, pragmatic trials, which she plans to use while working on the Dial Mag Trial, which is a pragmatic cluster randomized trial that asks whether hemodialysis centre-wide adoption of a higher concentration of magnesium in hemodialysate alters patient health. Thanks to Ms. Killin’s contributions and that of a broader KDT team, both trials have the capacity to bring significant impact to the practice of hemodialysis and the quality of life for persons living with kidney disease.
Ms. Killin’s enthusiasm for clinically impactful projects has driven her to become a pivotal member of the KDT team. The collaborative working environment and rich learning experience that KDT provides to staff have built a strong, tightly-knit community. Ms. Killin, because of her kindness, her love for learning, and her desire to build change in healthcare, is an integral part of that community. KDT is honoured to have Ms. Killin on our team and can’t wait to see how far her work will reach.
Data and Linkages
Linked Data Projects
KDT researchers regularly link new datasets to the ICES data holdings to expand the scope and breadth of their research. This year, the following project linked data with ICES databases:
- Gurleen Sahi at Western University linked height and weight values to evaluate obesity in CKD patients for his project “Prevalence and characteristics of patients with obesity across the kidney disease spectrum.”
KDT is proud to share recent publications from our ongoing projects that linked data with ICES:
- SARS-CoV-2 testing, infection and outcomes among Ontario physicians: a descriptive population-based cohort study - Liu CW, Jeyakumar N, McArthur E, Sontrop J, Myran D, Schwartz K, Sood M, Tanuseputro P & Garg A.
- Pre-Operative kidney biomarkers and risks for death, cardiovascular and chronic kidney disease events after cardiac surgery: The TRIBE-AKI study – Vasquez-Rios G, Moledina D, Jia Y, McArthur E, Mansour S, Thiessen-Philbrook H, Shlipak M, Koyner J, Garg A, Parikh C & Coca S, for the TRIBE-AKI Consortium.
Interjurisdictional Collaboration
KDT actively collaborates with researchers across the globe to improve the quality and scope of their research and expand the impact and reach of scientific findings. This allows researchers to use ICES administrative health data while developing a growing knowledge base that better informs health care and policy worldwide.
KDT welcomed the opportunity to support the following new inter-jurisdictional collaborations:
- Conor Judge at Galway University Hospital, Galway, Ireland – Protocol Development for “Sodium fOr diaLysis oUTcome rEduction (SOLUTE) Trial.”
- Pavel Roshanov at London Health Sciences Centre, London, Ontario – “Post-discharge after Surgery Virtual Care with Remote Automated Monitoring Technology (PVC-RAM) Trial - Clinical and Health Utilization Outcomes (PVC-RAM-Clinical and Health Utilization Outcomes).”
- Amit Garg at London Health Sciences Centre, London, Ontario – “Preliminary investigation of in-centre chronic hemodialysis to guide the Dial Mag Canada trial study design.”
We are happy to share a recent publication that resulted from KDT’s ongoing inter-jurisdictional collaborations: Kidney function and the comparative effectiveness and safety of direct oral anticoagulants versus warfarin in adults with atrial fibrillation: a multicenter observational study, by Min Jun (University of New South Wales, Syndey, Australia) et al.
The findings from these collaborations expand the reach of our Ontario-based datasets. We will continue our collaborations with international researchers in their endeavours to answer questions that improve health care and policy worldwide.
Organizational Health
KDT’s environment makes it possible for us to successfully support our community. This success is heavily dependent on the wellbeing of our staff. That is why KDT focuses on policies, processes, and concerns that affect the staff’s ability to perform at their best. Since April 2022, KDT has nurtured our organizational health and fostered an environment allowing our staff to produce their best work. For example, we:
- Celebrated at our annual in-person event where we shared program updates and wins, discussed ways to improve, built team rapport, and afterwards met at the Program Lead’s home for a casual get together. Insights, included:
- Staff's preference to adopt a virtual environment – a team-wide policy is currently being developed.
- A request for a compensation review – KDT management is part of an LHSC working group that is performing a compensation review.
- Celebrated staff and community achievements through the continued publication of the KDT bulletin.
- Provided 376 hours of release time and $2,167 in funding for professional development via:
- SORA TABA Workshop
- Specialist Knowledge Translation Training
- Current Developments in Cluster Randomized Trials & Stepped Wedge Designs
- Gardener’s Grove 2023 Conference
- Strengthened internal relationships and processes through working groups for:
- DCP analytic enhancements – Danielle Nash, Steph Dixon, Jennifer Reid, and Yuguang (Greg) Kang developed resources to improve clarification and standardization for methodology and analytics across DCPs.
- File storage and sharing – Melody Lam, Sarah Bota, Bin Luo, and Shane Kilburn organized our shared resources.
- Project resources – Sarah Bota, Danielle Nash and Shane Kilburn developed a resource centre for KDT staff to support best practices.
- DCP analytic enhancements – Danielle Nash, Steph Dixon, Jennifer Reid, and Yuguang (Greg) Kang developed resources to improve clarification and standardization for methodology and analytics across DCPs.
- Provided four additional paid days off for all staff; recognizing the hard work being done and the dedication of our great staff.
Our team grew a great deal this year with the addition of Sheikh Abdullah, Samar Almadhoun, Taylor McLinden, and Kathryn Stirling. In addition, Danielle Nash successfully completed of her post-doctoral work and Bin Luo was promoted to senior analyst.
Thank you to all our staff for your support of each other and our community, while diligently striving to go above and beyond. KDT wouldn’t be the successful program that it is without you!

Best Practices
This year, ICES introduced a network-wide $1,000 annual project fee for active projects. Projects active prior to April 1, 2023, will be charged quarterly. Projects activated after April 1, 2023, are in year 1 and will be subject to the first-year cost of $1,000; subsequent years will be prorated.
- Projects are active from analytics through publication,
- A $1000 invoice will be issued (covers 1 year of activity),
- After the first year, active projects are $250 each quarter,
- Once published, PIs must request that fees stop.
To make this process work, please continue to communicate deadlines, expected timelines, and any known interruptions to your schedule as soon as you can.
Strengthening Partnerships
Ontario Renal Network
On October 19, 2022, the Ontario Renal Network (ORN) (part of Ontario Health) and KDT held a partnership meeting, which brought together ORN leadership, KDT investigators, patient and caregiver partners, and staff from both the ORN and KDT. As a result of the ORN’s long-standing relationship with the ICES KDT team, the meeting focused on increasing collaboration. During the meeting, ORN leadership presented their current and emerging research priorities across key clinical areas and engaged the audience in a dialogue about mutual areas of research interest, ongoing research projects, new project ideas, and future areas for collaboration. The meeting successfully built a platform for partnership and initiating dialogue between investigators and the ORN for future collaborations.
Furthermore, in support of its nine-year relationship with the ORN, this year KDT has collaborated on projects such as:
- Chronic kidney disease quality-of-care indicators for First Nations and Métis People (participatory community-based research directed by Indigenous partners and their communities)
- Determining whether the implementation of routine symptom assessment and management improves the health outcomes of people receiving dialysis
- Characteristics and outcomes of patients receiving virtual versus in-person MCKC care during the COVID-19 pandemic period
- Feasibility and efficacy of potential indicators to measure program and provincial performance in treating patients with early CKD
- Determining the prevalence of CKD in Ontario across different demographic and clinical characteristics, and identifying gaps in early CKD care
We are operating under the second year of our five-year $1.14M ORP3 CIHR grant, which focuses on patient-driven priority research projects done in collaboration with KDT investigators, patient partners, and the ORN. Pursuant to this grant, KDT’s incredible progress has resulted in 16 active projects, from which five manuscripts are being prepared for publication and one has already been published. KDT plans to maintain this momentum over the next three years, focusing on high-impact projects that result in real improvements to patient care. Please reach out to Danielle Nash if you have an impact story to share.
We also organized several activities to strengthen and build the ORN’s learning healthcare system. The analytics teams at both organizations met regularly to build consensus and validate technical details of the data and active projects. KDT community members presented to the ORN’s Provincial Leadership Table.
Trillium Gift of Life Network
In continued partnership with the Trillium Gift of Life Network (TGLN, part of Ontario Health) and its research related to organ donation and transplantation, we advanced several projects focused on creating impactful research, such as:
- Quality indicators for access to kidney transplant - transplant referral – PI: Kyla Naylor
- Outcomes of patients with Alcohol-associated Liver Disease (ALD) declined from Ontario’s ALD pilot program – PI: Jennifer Flemming
- COVID-19 in solid organ transplant recipients – PI: Greg Knoll
- Effectiveness of first, second, and third COVID-19 vaccine doses in solid organ transplant recipients: a population-based cohort study from Canada - PI: Kyla Naylor
This collaboration resulted in a publication that demonstrated that COVID-19 vaccine effectiveness in solid organ transplant recipients is lower than the general population, however, vaccine effectiveness improved following a third dose. We anticipate this partnership yielding additional impactful research to support improving health and healthcare for persons living with kidney disease. In the future, TGLN and KDT are developing new proposals and plans to fund important work.
Featured Research
COVID-19 Projects and Publications
Because of the ongoing international spread and evolution of COVID-19, many researchers remain focused on determining the virus’ impact, long-term consequences, and effectiveness of the COVID-19 vaccine among persons living with CKD.

There are several active COVID-19-related projects within the KDT program examining different topics, including:
- COVID-19 vaccine effectiveness for solid organ transplant recipients – PI: Kyla Naylor
- COVID-19 vaccine effectiveness for persons living with kidney disease, in the pre-dialysis population, and those on dialysis – PI: Matthew Oliver
- The impact of COVID-19 on patients receiving maintenance dialysis – PI: Kyla Naylor
- The impact of COVID-19 on outcomes for people with kidney disease – PI: Steph Dixon
- Trends and outcomes of virtual multi-care kidney clinic care during the COVID-19 pandemic – PI: Ann Young
- Long-term outcomes of COVID-19 in the maintenance dialysis population – PI: Matthew Oliver
- The impact of the novel COVID-19 outbreak on physicians – PI: Amit Garg
As a result of these efforts, several manuscripts have been published and their findings are already impacting health care and health care policies for persons living with kidney disease. This research found:
- The rate of physicians seeking outpatient care for mental health and substance use increased on average by 13% during the first 12 months of the pandemic compared to the previous 12 months.
- COVID-19 vaccine effectiveness in solid organ transplant recipients was found to be lower than the general population, however, vaccine effectiveness improved following a third dose.
- During the COVID-19 pandemic, the shift in primary health care from in-person to virtual care led to a drop in carbon dioxide emissions owing to reduced patient travel and millions of dollars saved in parking, gasoline, or public transit costs. These benefits are likely to continue as virtual care is maintained as part of the health care system.
Our program remains dedicated to helping researchers study COVID-19 and promoting their work to improve COVID-19-related health care and policy. If you would like more information regarding COVID-19-related research being conducted, please contact Kyla Naylor.
Dial-Mag Trial
Despite dialysate being a critical component of hemodialysis, little evidence is available to guide the optimal dialysate formulation. New research suggests that a higher dialysate magnesium concentration may benefit patients.
The Dialysate Magnesium (Dial-Mag) Trial is a multi-centre pragmatic cluster randomized trial being conducted in 156 dialysis units located across Alberta, British Columbia, Manitoba, and Ontario. The trial team seeks to determine the best dialysate magnesium concentration for the health of patients on dialysis.
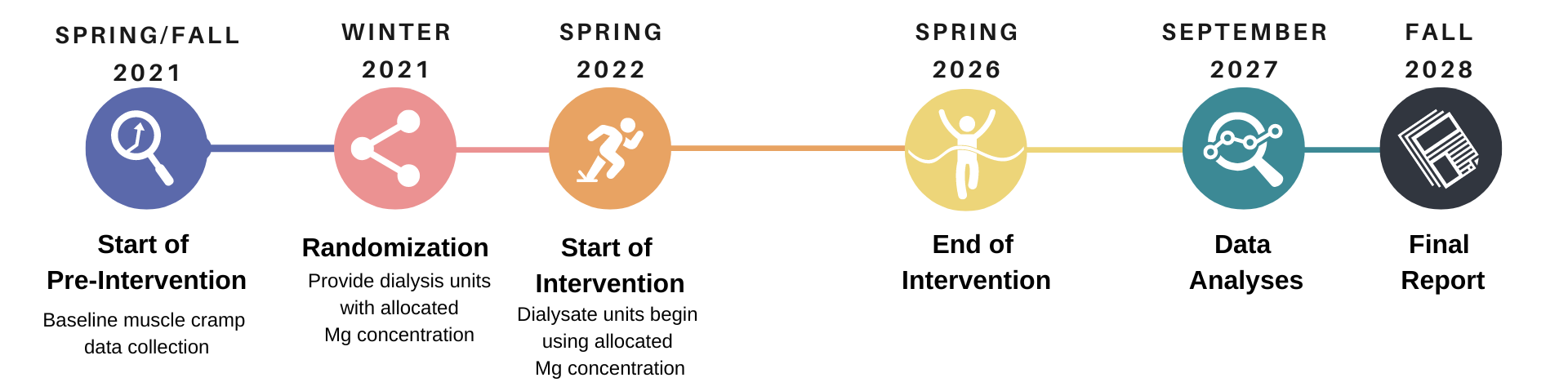
The trial is anticipated to include more than 25,000 participants and 15.6 million total dialysis sessions over four years. The Dial-Mag researchers hope to determine whether the serum magnesium concentrations have an impact, and the optimal concentration of dialysate magnesium concentrations for improved patient health.
MyTEMP
Conventionally, dialysis centres have provided hemodialysis using a standard dialysis fluid temperature of 36.5°C for all patients. Based on preliminary evidence that using a cooler dialysis fluid may have cardiovascular benefits, a growing number of centres are using a cooler temperature of 36°C or lower inpatient care.
The MyTEMP trial was conducted to examine whether providing dialysis using a cooler dialysis fluid as a centre-wide policy reduced the risk of cardiovascular-related hospital admission or death compared with using a standard temperature of 36.5°C. The MyTEMP trial team was led by Dr. Amit X. Garg, ICES KDT program lead and a nephrologist at the London Health Sciences Centre and professor of medicine, epidemiology and biostatistics at Western University.
The trial was conducted from 2017 to 2021 in 84 hemodialysis centres across Ontario and used several innovative and pragmatic methods, including:
- Integration of the intervention into routine care with minimal to no healthcare disruption.
- Participation of all patients at the 84 centres.
- Use of routinely collected data from existing administrative health data sources to reliably assess outcomes.

MyTEMP is the largest trial of maintenance hemodialysis published to date, including over 95% of patients receiving hemodialysis in Ontario during the trial period, totaling more than 15,000 patients who had more than 4.3 million dialysis treatments.
MyTEMP’s Findings
The MyTEMP team examined how many patients had cardiovascular-related hospitalizations or deaths in each group and determined that adopting a centre-wide policy of personalised cooler dialysate versus a standard dialysate temperature of 36.5°C did not reduce the risk of major adverse cardiovascular events or death. In addition, patients in the personalised cooler dialysate group were more likely to report feeling cold on dialysis than respondents in the standard-temperature group.
Based on the trial’s findings, dialysis centres that are currently providing cooler dialysate as a centre-wide policy should consider using a dialysate temperature of 36.5°C as their standard for the comfort of their patients. The role of cooler dialysate in the management of specific types of patients who have frequent episodes of low blood pressure during dialysis now needs to be clarified in future studies.
The trial resulted in three publications:
EnAKT LKD Trial

For persons living with kidney failure, kidney transplantation offers patients a longer and better quality of life compared to dialysis. Transplant recipients can receive a kidney from a deceased or living donor. A transplant from a living donor offers many advantages, including superior graft and patient survival, shorter wait times, and lower health care costs.
In partnership with patients, and health care providers and with health administrators at TGLN and the ORN, the Enhance Access to Kidney Transplantation and Living Kidney Donation intervention was developed. The intervention was implemented from November 1, 2017, to December 31, 2021, and was a province-wide pragmatic, registry-based, cluster-randomized clinical trial. We evaluated the effect of a multi-component quality improvement intervention designed to help eligible patients complete key steps toward receiving a kidney transplant.
The EnAKT LKD Trial included 26 CKD programs and six adult transplant centres across Ontario, which provide care for approximately 20,000 potentially transplant-eligible patients with advanced CKD. These centres were randomized, with 13 CKD programs receiving usual care and 13 CKD programs being set with a multi-component quality improvement strategy included four main components:

* The Transplant Ambassador Program is a patient-led program that connect patients who have kidney failure to individuals who have successfully received a kidney transplant or donated a kidney.
During the trial, the core operations group met more than 100 times, the 13 CKD programs established a local quality improvement team, developed a charter, and teams met regularly to review and improve transplant performance. More than 1700 patients completed the Explore Transplant Ontario education program and transplant ambassadors recorded more than 5700 interactions with patients with CKD and potential living kidney donors.
The trial has resulted in four publications so far:
- The EnAKT Protocol for Process Evaluation
- The EnAKT Quality Improvement Intervention Protocol
- The EnAKT Statistical Analysis Plan
- Partnering with Patients
Full EnAKT trial results will be publicly available in the coming months.
ICES KDT Community
ICES KDT Scientists
All ICES projects require active participation by an appointed ICES scientist to ensure that ICES policies and procedures are followed. As such, our scientists provide pivotal support for all KDT’s projects and the broader KDT community.
Thank you to our scientists for their dedication and hard work in making KDT a success:
*ICES Scientists with KDT as a secondary affiliate rather than primary affiliation.
For information on how to become an ICES scientist or to collaborate with an ICES KDT scientist, please go to https://www.ices.on.ca/join-our-research-community/.
ICES KDT Trainees
ICES KDT provides educational opportunities for graduate and post-graduate trainees interested in conducting research focused on kidney, dialysis, and transplantation health and policy. The program provides these trainees with access to a large collection of Ontario administrative and clinical databases to help them meet the thesis, practicum or course requirements of their graduate degrees. Since December of 2012, KDT supported the work of 37 students, of which 18 are currently active. We are proud to report that between April 1, 2022, and March 30, 2023, the following KDT trainees successfully met their trainee requirements and were offboarded from ICES:
- Stephanie Kim – supervisor Andrew Simpson
- Fatemeh Ahmadi – supervisor Amit Garg
In addition, our own Danielle Nash successfully completed her post-doctoral work!
For information on becoming an ICES student, visit https://www.ices.on.ca/join-our-research-community/become-an-ices-student/.
