
For Ontario’s population of approximately 14 million people, advanced chronic kidney disease (CKD) care is provided by 26 CKD programs, which treat approximately 24,000 Ontarians each year who are approaching the need for dialysis or receiving maintenance dialysis.
Due to the poor outcomes and the complex care that patients receiving dialysis need, they are often excluded from clinical trials. In addition, nephrology has the fewest clinical trials conducted of any medical discipline and many nephrology trials fail to provide conclusive evidence because of small sample sizes, poor patient representation, missing data, and poor intervention adherence. As such, hemodialysis care is largely based on expert opinion rather than robust trial-based evidence.
Since 2017, our team has started to address this gap by conducting several large-scale pragmatic randomized controlled trials (RCTs) to improve hemodialysis care. With the support of the Canadian Institutes of Health Research (CIHR) Strategy for Patient-Oriented Research (SPOR) innovative Clinical Trials (iCT) initiative, we have successfully completed one RCT, are currently conducting three additional RCTs, and have several additional RCTs at various stages of development, all to generate robust evidence to guide care which improves patient outcomes and healthcare provision.
Pragmatic trials embed the trial into routine healthcare, allowing all patients in the clinical setting to participate in the trial, resulting in a trial sample that is representative of the population of interest and highly generalizable trial findings. This type of trial design allows our trial teams to undertake RCTs by supporting the development and testing of innovative interventions using new methodologies and designs, integrated with SPOR’s principles, and to build capacity for research through training.


MyTEMP and MyTEMP PRO
The MyTEMP trial was conducted to examine whether providing dialysis using a personalised cooler dialysis fluid reduced the risk of cardiovascular-related hospital admission or death compared with using a standard temperature of 36.5°C.
The trial was conducted in 84 of Ontario’s 97 hemodialysis centres and was the largest trial of maintenance hemodialysis published to date, including over 95% of patients receiving hemodialysis in Ontario during the trial period, totalling more than 15,000 patients who had more than 4.3 million dialysis treatments.
The trial resulted in the following publications:
The MyTEMP team examined how many patients had cardiovascular-related hospitalizations or deaths in each group and determined that adopting a centre-wide policy of personalised cooler dialysate versus a standard dialysate temperature of 36.5°C did not reduce the risk of major adverse cardiovascular events or death. MyTEMP PRO was a sub study focused on examining patient self-reported symptoms and found that patients in the personalized cooler dialysate temperature group were more likely to report feeling uncomfortably cold than in the standard group. The role of cooler dialysate in the management of specific types of patients who have frequent episodes of low blood pressure during dialysis now needs to be clarified in future studies.
Results from the MyTEMP trial were widely publicized in several news releases, newsletters, and bulletins, including Western News, Can-SOLVE CKD, Rethinking Clinical Trials, Med Page Today, Renal Interventions, and London News Today, and were presented at the ASN 55th Annual Meeting and Scientific Exposition, ORN Provincial Leadership Table, ICES Evaluative Sciences Rounds and PHRI Grand Rounds. The results were also distributed to dialysis units and patients receiving dialysis via posters, patient handouts, and a lancet editorial.

Dial-Mag Canada
Magnesium is an essential element that supports heart, muscle, and bone health. During dialysis, some magnesium is removed from the blood. Dialysis fluids (dialysate) has magnesium added to replace the magnesium removed during the dialysis process.
The concentrations of magnesium in dialysate can vary depending upon the dialysate manufacturer and the prescribing physician’s orders. In smaller trials, lower serum concentrations of magnesium were associated with a higher risk of cardiovascular disease, muscle cramps, and fractures.
The Trial
The Dialysate Magnesium (Dial-Mag) Trial is a large-scale randomized pragmatic clinical trial focused on determining whether different concentrations of magnesium in dialysate are alter outcomes that matter most to patients and their providers. Dial-Mag is currently running in four Canadian provinces in 155 hemodialysis centres. Through this trial, we hope to determine the optimal concentration of dialysate magnesium for patient health.

As part of the trial, the hemodialysis centres were randomized into two groups: the first set of units in general uses a dialysate magnesium concentration no more than 0.5 mmol/L, and the second administers a dialysate magnesium concentration of 0.75 mmol/L. Participating centres in general provide the trial assigned dialysate magnesium concentration to all patients receiving care in their centre. Patients’ health outcomes are being gathered throughout the duration of the trial.
Dial-Mag has been embedded into routine hemodialysis care, with the goal of minimizing disruption to healthcare workflow. Dial-Mag will leverage routinely collected data that is already being captured as a part of standard healthcare practice and every six months will collect data on the severity of muscle cramps that patients experience. We anticipate sharing results on more than 8 million hemodialysis sessions by the of Fall 2027.
RESOLVE
The Randomized Evaluation of SOdium dialysate Levels on Vascular Events (RESOLVE) trial is an ongoing collaboration with The George Institute for Global Health in Australia, involving more than 300 hemodialysis centres worldwide. This multi-national trial aims to establish whether treatment with a dialysate sodium concentration of 137 mmol/L, rather than 140 mmol/L, reduces cardiovascular events and death in hemodialysis patients.
To date, 20 Ontario hemodialysis centres are actively participating in the trial, having been randomly allocated to a dialysate sodium concentration of 137 mmol/L or 140 mmol/L. We are finalizing the analytic plan to aggregate data from Canada with global data. The trial started in 2017 and follow-up is ongoing.

PREFERRED-1
The PRevEnting FracturEs in REnal Disease 1 (PREFERRED-1) pilot trial, which is currently underway, hopes to reduce the fragility fracture risk faced by patients on hemodialysis. The PREFERRED-1 team, led by Dr. Kristin Clemens, is comprised of endocrinologists, nephrologists, geriatricians, researchers, and patients. The team is investigating the effectiveness of denosumab, a monoclonal antibody commonly used in the treatment of osteoporosis.
During this pilot trial, the trial team will determine how best to embed the trial into routine care at dialysis units, and whether recruiting an adequate number of patients into a large trial is feasible. The trial concept was presented at the 2023 Gardener’s Grove Conference. Based on the current experience with the pilot trial, the team is considering whether to pivot to examine a dialysis unit-level fracture prevention strategy (which may include the use of denosumab), rather than on randomly allocating individual patients to the use of denosumab (yes vs. no).


Dial-Bicarb
The optimal concentration of baking soda in dialysis fluids (dialysate bicarbonate) for best patient outcomes is currently unknown. Guidelines suggest that providers should use a pre-dialysis blood bicarbonate concentration target ≥ 22 mmol/L, but these guidelines are based on weak evidence. Because of this, some dialysis units deliver a single bicarbonate concentration to all patients while others adjust the concentration, based on the patient’s pre-dialysis blood analysis results. In those that provide a single bicarbonate concentration the value differs, from 32 to 40 mmol/L.
Research conducted in small trials suggests correcting metabolic acidosis has a positive effect on nutrition, muscle mass, insulin sensitivity, and bone metabolism. However, large observational studies show that a higher dialysate bicarbonate concentration could be associated with increased mortality.
The Dial-Bicarb Trial Protocol is a large-scale pragmatic RCT focused on determining which dialysate concentration of bicarbonate is best for patient health. Patient partners are heavily involved in developing the consent process and helping to refine the intervention. The trial team is also collaborating with the research ethics board at Western University, to develop an acceptable method of streamlined patient consent to be used in this trial.
The proposed Dial-Bicarb Trial was one of six RCTs chosen to be presented at the Gardener’s Grove 2023 Conference in March 2023. The trial received a grant from the Accelerating Clinical Trials Canada Consortium and in August 2023, Drs. Amber Molnar and Samuel Silver were awarded a $1.7 million grant from CIHR to fund the Trial.
Sodium fOr diaLysis oUTcome rEduction (SOLUTE Trial)
Maintenance of blood pressure in persons on hemodialysis is vital but can be very difficult. . Observational studies have shown a positive association between a lower concentration of dialysate sodium and lower blood pressure, lower interdialytic weight gain and lower anti-hypertensive medication use. However, a prior study showed no difference in left ventricular mass index, despite differences in interdialytic weight gain.

The SOLUTE Trial concept, led by Dr. Conor Judge, wishes to compare a zero-sodium gradient (0 mmol/L) with a fixed dialysate sodium of 138 mmol/L in a cluster randomized controlled trial, essentially personalizing the sodium concentration to the patient. The trial will evaluate the effectiveness on the primary outcome of cardiovascular events.
To aid with data collection for pragmatic trials, the SOLUTE team (based at the University of Galway) is developing a multi-university, multi-agency Irish Renal Registry to help provide the governance, technical, consent and accessibility for use of routinely collected data. This proposed database would run as a parallel database to ICES and aid in data collection and analyses of trials across Ireland and Ontario.
This proposed trial was presented by Dr. Judge at the 2023 Gardener’s Grove Conference in March 2023. The trial concept is under development.
Finding the Right Blood Pressure Target for Patients on Dialysis
High blood pressure in people receiving hemodialysis is known to contribute to cardiovascular morbidity and mortality through excess strokes, heart failure, left ventricular hypertrophy, and arrhythmias, whereas low blood pressure can lead to cramps, myocardial stunning, and cerebrovascular ischemia. However, despite, blood pressure management being a cornerstone of multidisciplinary rounds, the optimal blood pressure target for people on hemodialysis is unknown.
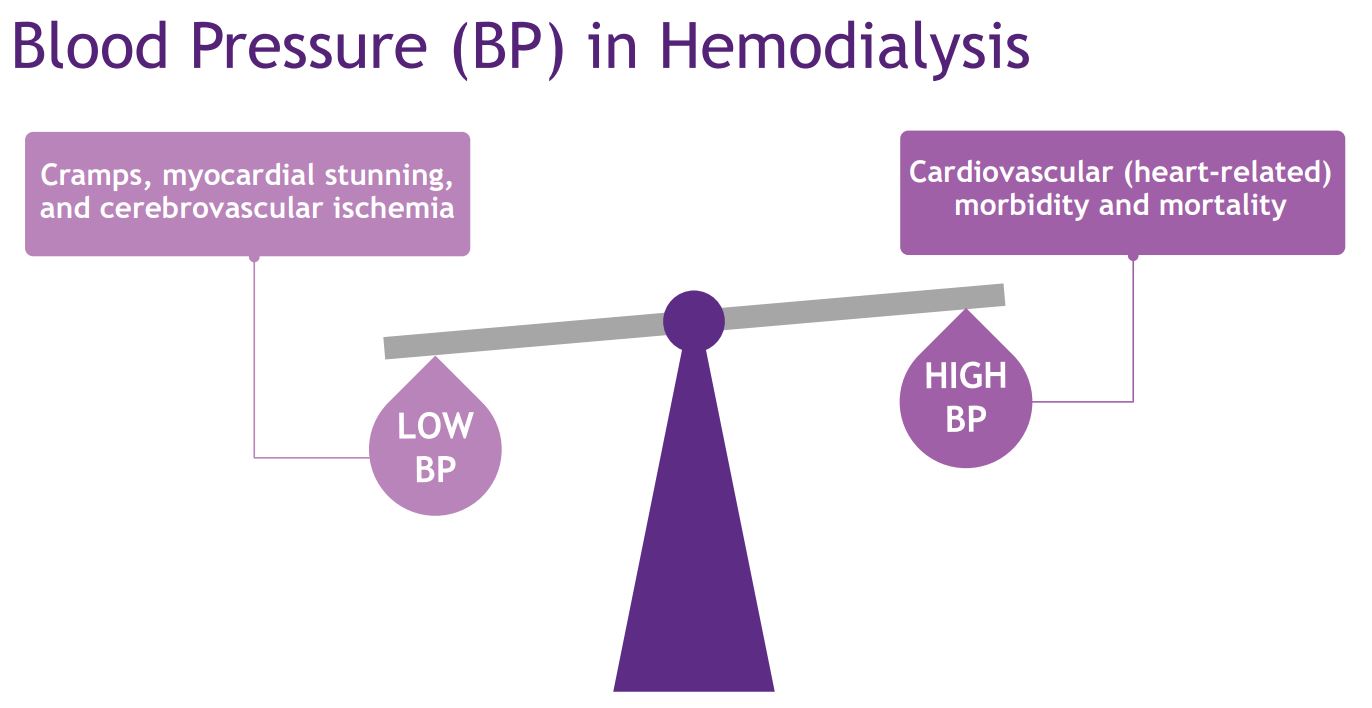
Finding the “right” blood pressure to target can have a major impact on patient lives. Dr. Navdeep Tangri and Dr. Karthik Tannankore are developing the concept for a large, pragmatic, cluster randomized trial targeting a lower versus higher blood pressure target in in-centre hemodialysis units in Canada. Their initial steps towards this trial are to determine the current treatment practices in Canadian hemodialysis units – hypothesizing that the target blood pressure in Canadian hemodialysis units is highly variable and poorly implemented, in turn setting the stage for a lower versus higher blood pressure target protocol tested in a future trial. The ultimate goal is to deliver a definitive randomized trial for blood pressure targets in the hemodialysis population.
This proposed trial was presented by Dr. Tennankore at the 2023 Gardener’s Grove Conference in March 2023. This trial is still in development.

KidneyCare Outreach
KidneyCare Outreach is an initiative aimed at ensuring all Ontarians with evidence of kidney disease receive the best possible care. Much like cancer screening service outreach conducted to encourage early detection of cancer, the KidneyCare research team will connect with Ontarians who may have undiagnosed kidney disease or are at risk for worsening disease and would benefit from seeing a nephrologist.
The team will identify these individuals based on prior lab results and health services used and paid for by OHIP. Individuals who are identified as possibly having undiagnosed kidney disease or are at risk for worsening disease will be contacted to inform them of these findings and gauge their willingness to participate in the initiative. The participants’ current kidney health will then be assessed through new blood and urine testing and a virtual visit with a study nephrologist. Any participants needing ongoing care will be referred to a nephrologist in the existing healthcare system. The vanguard phase of the initiative is an internal pilot trial testing the feasibility of this new approach at ICES.
The initiative is currently being developed with the hope of launching in 2024. The vanguard phase has received grant funding from the Accelerating Clinical Trials Canada Consortium and the New Frontiers in Research Fund Exploration stream. This initiative is being developed in collaboration with ICES and London Health Sciences Centre and is overseen by the Western University Research Ethics Board and a Data Safety and Monitoring Board.

The story of success can be seen in the extensive body of work published by dedicated investigators.
- Patient and caregiver involvement in a multicentre clustered hemodialysis trial
- Barriers and facilitators to healthcare professional behaviour change in clinical trials using the Theoretical Domains Framework: a case study of a trial of individualized temperature-reduced haemodialysis
- Major Outcomes With Personalized Dialysate TEMPerature (MyTEMP): Rationale and Design of a Pragmatic, Registry-Based, Cluster Randomized Controlled Trial
- MyTEMP: Statistical Analysis Plan of a Registry-Based, Cluster-Randomized Clinical Trial
- Personalised cooler dialysate for patients receiving maintenance haemodialysis (MyTEMP): a pragmatic, cluster-randomised trial
- Cultivating Innovative Pragmatic Cluster-Randomized Registry Trials Embedded in Hemodialysis Care: Workshop Proceedings From 2018
- A Higher Concentration of Dialysate Magnesium to Reduce the Frequency of Muscle Cramps: A Narrative Review
- Addressing feasibility challenges to delivering intradialytic exercise interventions: a theory-informed qualitative study
- Diabetes Management in Older Adults With Chronic Kidney Disease
- Addressing feasibility challenges to delivering intradialytic exercise interventions: a theory-informed qualitative study
- Magnesium and Fracture Risk in the General Population and Patients Receiving Dialysis: A Narrative Review
- Denosumab in chronic kidney disease: a narrative review of treatment efficacy and safety
- Ethical issues in pragmatic cluster randomized trials in dialysis facilities
- Reporting of key methodological and ethical aspects of cluster trials in hemodialysis require improvement: a systematic review
- Ethical Issues in the Design and Conduct of Pragmatic Cluster Randomized Trials in Hemodialysis Care: An Interview Study With Key Stakeholders
- Patient Partner Perspectives Regarding Ethically and Clinically Important Aspects of Trial Design in Pragmatic Cluster Randomized Trials for Hemodialysis
- A critical examination of informed consent approaches in pragmatic cluster randomized trials: Cory Goldstein PhD Thesis
- Machine learning algorithms to identify cluster randomized trials from MEDLINE and EMBASE
- Simple compared to covariate-constrained randomization methods in balancing baseline characteristics: a case study of randomly allocating 72 hemodialysis centers in a cluster trial
- Educational material for the NIH Collaboratory’s Living Textbook of Pragmatic Clinical Trials

Cultivating the next generation of clinical researchers is vitally important for the betterment of health and healthcare, the continuation of research, and the growth of knowledge and further research capacity. Thus, tending the seeds planted by new researchers and programs is vital for the continued success of our research program. We are pleased to showcase two seeds that have flourished into saplings.
Dial-Mag Bone Substudy
Individuals receiving hemodialysis have a five-fold increased risk of fracture compared to the general population. Higher serum magnesium concentration has been associated with a lower risk of fracture in patients receiving hemodialysis, and magnesium supplementation may decrease parathyroid hormone (PTH) concentrations.
Dr. Andrea Cowan, a nephrologist at London Health Sciences Centre, is leading a team that is developing a sub-study of the Dial-Mag trial to determine the effect of higher vs lower dialysate magnesium concentration on fracture risk, serum PTH concentration, and consequences of tertiary hyperparathyroidism in patients receiving hemodialysis.
As part of the Dial-Mag Trial, the Bone Substudy team will use administrative health database to track patients in the two groups of dialysis centres that were randomized to high or low dialysate magnesium concentrations over the four-year trial. The team will observe the primary outcome, the cumulative incidence of fracture between intervention and control groups, and secondary outcomes including the difference in parathyroid hormone level between the two groups over time, and a composite outcome of parathyroidectomy, cinacalcet use and escalation of calcitriol dose.
The team anticipates that PTH levels will be lower in the high magnesium group compared to the low magnesium group, with potentially lower rates of parathyroidectomy and cinacalcet use and a trend towards lower fracture rates.
The HDRN Pragmatic Trials Training Program

In January 2023, the Canadian Institutes of Health Research awarded $3.5M to develop the HDRN Canada Pragmatic Trials Training Program. This two-year virtual pan-Canadian training program will coach researchers through the complicated art of conducting pragmatic clinical trials. This two-year virtual pan-Canadian program will provide training to advanced learners across three streams: (1) future trial leaders (faculty-level trainees), (2) postdoctoral fellows, and (3) highly qualified personnel.
More than 90 excellent applications, which were due by December 22, 2023, were received and a pan-Canadian review process is currently ongoing. Learners will gain specialized knowledge and skills in pragmatic clinical trial methodology and health systems research, and access funding, mentorship, and networking opportunities .

Co-developing trials with patient partners is vital to the nourishment and growth of high-impact trials and trial success. As such, the growth and cultivation of new and long-lasting relationships with patient partners continue to be a priority. In 2023, we engaged with 15 patient partners from four provinces across seven trials. These patient partners engaged in all research stages, including helping identify research priorities, refining study plans, developing and implementing the trials, assisting with interpreting findings, developing patient-facing materials, and fostering knowledge transfer.
“As a patient partner, participating in the building of a trial is one of the most rewarding experiences of my long patient life. This work allows patients the opportunity to have a say and feel more in control of their own care, while making a difference for themselves and the patient sitting beside them at dialysis.”
-Brenden Cote, Patient Partner on the MyTEMP Trial
We appreciate all the work that our patient partners have done and look forward to continued collaboration. If you would like to engage with patients in your research and would like support, please contact [email protected].

The Schulich School of Medicine and Dentistry at Western University hosts an emerging clinical trial core facility, to encourage and support the advancement of high-impact clinical trials in healthcare. The platform was launched during a virtual workshop on April 30, 2022.
This facility is a unique researcher-support and training program that gives investigators, patients, clinicians, and healthcare administrators the knowledge, skills, and aid to prepare promising RCT interventions. The platform is also one of eleven clinical trial units named in the Accelerating Clinical Trials Canada Consortium.
The initial focus of this facility is on the conduct of large, pragmatic trials. It supports trial teams as they design, lead, and execute better, faster, and cheaper trials, ultimately, proving some interventions work, supporting their broad adoption in healthcare, and contributing to healthier people and societies.
Specifically, the stream offers several services to interested researchers, including:
- Protocol development and refinement
- Pilot trials and full trial execution
- Analysis of existing administrative data to guide trial conduct
- Administratively supporting partnerships
- Human resource, administrative, and governance planning for trials
- Grant planning, preparation, submission, and reporting
- Trial ethics
- Supporting inter-institution contracts
- Support to establish data and safety monitoring board
- Trial budget creation, monitoring, invoicing
- Trial management
- Communications
- Public preparation
- Supporting training of investigators and highly qualified personnel
By accessing the expertise and services provided by the Pragmatic Trials Stream, researchers will gain insight and support to test simple, scalable, sustainable, and streamlined real-world solutions.

From March 27-30, 2023, we held the Gardener’s Grove 2023 virtual conference, where we gathered a coalition of the willing from researchers, patients, healthcare providers, and other stakeholders to discuss exciting new ideas for patient-centered research. This conference is the continued legacy of the inaugural Gardener’s Grove conference, held in 2018.
The conference, which focused on increasing knowledge and awareness of pragmatic large-scale randomized controlled trials embedded into routine hemodialysis care, consisted of 10 Educational Sessions discussing all aspects of pragmatic RCTs and six Panel Presentations. Several trial ideas presented at both the 2018 and 2023 conferences have since been implemented as large-scale trials: an exciting legacy and something for the participants to be proud of.
Gardener’s Grove will be back in 2026, where we will again bring together a talented group of individuals to present exciting new ideas for pragmatic trial opportunities and to discuss patient-centered research.
We look forward to seeing you there!
